Carriage of Fertiliser Cargo

Background
Fertilisers are synthetic or natural, organic or inorganic materials used to supply nutrients vital for plant growth. Plants uptake nutrients dissolved in water through their roots, with nitrogen (N), potassium (K) and phosphorus (P) being the primary elements required for plant growth. Therefore, fertilisers find its use mainly in agriculture. The estimated global fertiliser market is expected to increase to 188.18 billion USD by 2024, this is likely to be accompanied by an increase in fertiliser distribution across the globe.
Commercial fertilisers can be composed of varying compositions; standard nomenclature for combined NPK fertilisers states the composition in the order of N, P and K followed by the percentage of each constituent. For example, NPK 15-15-15 consists of 15 % N, 15 % P and 15 % K. Commonly shipped fertilisers include urea, ammonium nitrate and potassium nitrate.
Although common types of NPK fertiliser have known compositions, the numerical values do not necessarily determine what the chemical entities in the mix are. Therefore, knowing that a fertiliser is a given NPK does not tell whether it may have hazardous properties. The most likely hazardous ingredient of an NPK is ammonium nitrate, and many NPK mixes do contain this. There are schedules in the IMSBC and IMDG Codes for fertilisers containing ammonium nitrate. Potential hazards include being an oxidising agent, self-heating, emitting toxic fumes, and undergoing self-sustaining decomposition.
This article explains how fertilisers are made and shaped as well as the issues that can arise during sea voyage.
How Fertilisers Are Shaped
Fertilisers are most commonly in prill or granule form. Importantly, the spherical shape and free-flowing nature allows them to be utilised in typical gravity fed field distribution equipment in the feilds.
Prills and granules exhibit differences in properties, some of which are relevant for transport and storage claims. It is worth briefly looking at how they are produced.
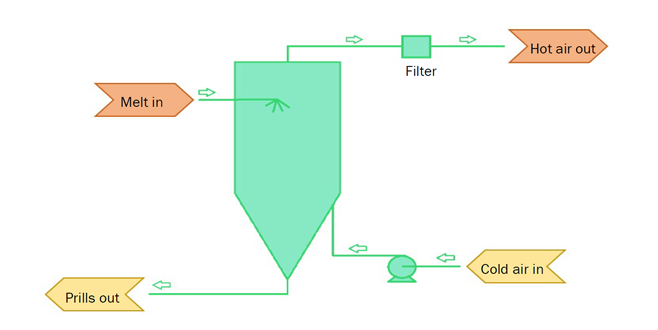
Prilling forms reasonably uniform spherical particles by forcing liquid through either a nozzle or a rotating bucket to form a small jet. This jet breaks up into individual liquid melt droplets that are cooled and solidified by freefalling in a ‘prill tower’ (Figure X), forming 0.5-4 mm spherical prills. Often, fans are added to the bottom of the tower to provide an upward steam of cool air.
There are two types of granulation; wet- or dry. Wet granulation is more common, with rotary drum granulators being the most often used method. Here, a mist of melt and binding agent droplets are fed into a high-speed rotating drum; small particles absorb the mist, growing to form larger granules of the desired size. The high temperature within the drum dries the granules.
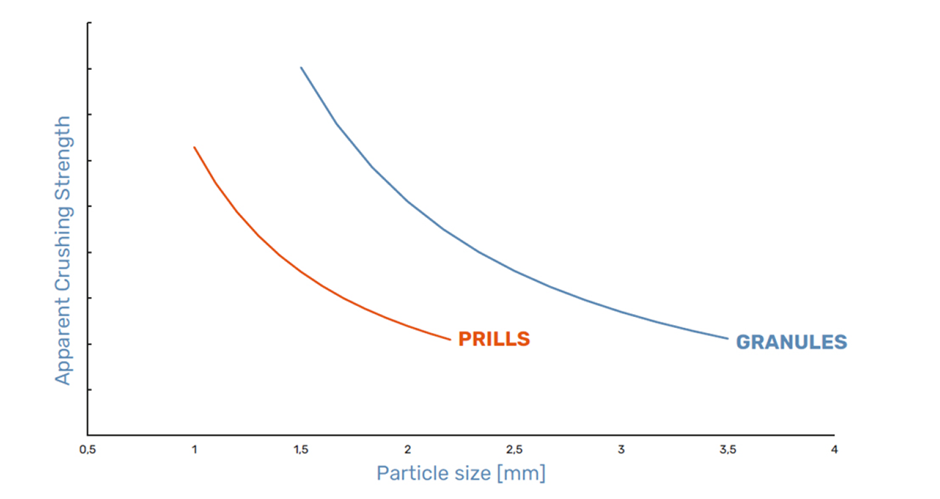
Prills and granules have differing physical and mechanical properties, with the key differences being in their resistance to crushing, i.e. hardness, and their size. The graph below shows the difference in hardness between prills and granules, depending on particle size; granules are shown to be harder and hence more resistant to crushing than prills, regardless of size.
Regardless of whether the fertiliser are prills or granules, the end product should meet these criteria:
- Consistent size
- Free from crushed granules and dust
- Easily spread
- Quickly dissolved
- Contaminant free
Caking in fertilisers
Between production and use in soil, fertilisers may need to be stored for long periods, and it is essential they remain free-flowing during this time. Under certain conditions, lumps or agglomerates (caking) can form in fertilisers- as fertiliser usage relies on their free-flowing nature, it is important to prevent caking. The contacts formed between fertiliser particles can provide an indication of the cause of caking. We point out that ‘free-flowing’ is different for fertilisers than, for example, grain, in that it is largely inevitable that some fertiliser compaction (adhesion contacts) to form freestanding cliffs within the stow will occur. These cliffs are easily collapsed by normal handling during discharge operations of the cargo, for example, using a grab.
The types of contacts that can form between fertiliser particles are detailed below. It is noteworthy that adhesion contacts, i.e. reversible compaction is distinct from hard caking, with the latter being irreversible.
Adhesion contacts, or capillary adhesion, is a relatively weak attraction between the in-contact molecule surfaces. Pressure exerted on the fertiliser particles in bulk can result in adhesion bound particles (i.e. compaction). Typically, adhesion contacts are easily reverted to a free-flowing state with minimal handling.
Liquid contacts are caused by fertiliser wetting or moisture vapour absorption. The saturated solutions formed between the wetted particles stick them together. As this liquid is mobile, the ‘sticking’ of the fertiliser particles is relatively easily broken. However, when the wetting is severe, phase contacts will be achieved.
Phase contacts, or salt bridges, are crystal bridges that form between fertiliser particles. These crystal bridges form due to dissolution/ recrystallisation and/or thermal effects. These salt bridges are often the most troublesome form of caking, resulting in hard caking that cannot be broken apart easily.
A wide range of factors, both external and inherent to the fertiliser, can affect the propensity for a cargo to cake, these are discussed briefly below.
Moisture Content at production influences the extent of caking, with higher moisture contents, even if they are within contractual specifications, being more prone to caking. The extent of drying required to limit the chances of caking depends on properties such as composition, morphology, size, and hardness.
The uniformity, size and crushing strength of fertilisers also impacts their likelihood to cake. Fertilisers with a small range in particle size, high crushing strength and larger sizes are less prone to caking due to reduced specific surface area for contact between particles, and decreased likelihood of breaking into smaller particles. Impurities within the fertiliser can also affect caking tendency, for example, iron and aluminium impurities decrease the propensity of caking in diammonium phosphate fertilisers.3
Moisture uptake during storage and handling prior to and during loading is another point of consideration. Moisture uptake is a particular risk during loading when all the cargo becomes exposed for at least some time on the conveyor. All fertilisers are hygroscopic, meaning they absorb moisture from the air, this occurs above the critical relative humidity (RH), which differs for individual fertilisers. RH is the amount of moisture in the air relative to the maximum the air can contain at any one temperature. For example, with urea, it is common practice to suspend loading at times of high atmospheric humidity, usually above 75 %- 80 % RH. It is also critical that fertilisers are not exposed to moisture during transportation.
Anti-caking agents are often added to fertilisers to minimise caking. These anti-caking agents are usually liquid or powder coatings that act as a barrier to prevent caking and can control the absorption of moisture by creating a hydrophobic barrier. They can also act as a physical barrier between particles or weaken bonds that may have formed between particles. Powder coatings can cause dust issues. Anti-caking agents can also be so-called ‘conditioning agents’, which improve the crushing strength and decrease dust formation. In the past, we have dealt with issues related to incorrectly or incompletely treated fertilisers.
Inappropriate ventilation can enhance caking, which can result in surface crusting from moisture uptake from humid ventilating air and/or caking in the top layer from excessive cooling. The respective IMSBC Code schedules for all bulk fertilisers specify that they shall not be ventilated during the voyage.
Temperature gradients can enhance caking if there are large temperature differences between load port and discharge port, resulting in caking along the periphery as and when cargo cools down. Similarly, if different production batches of fertilisers of dissimilar temperatures are loaded, this can also produce temperature gradients within the stow.
Fertiliser cargo issues
We often come across two main issues with fertiliser, both of which are usually caking, but the first being the master observing caking during loading and the second being caking from whatever causation at outturn.
More frequently we are dealing with bulk fertilisers rather than bagged, and often shippers are bringing bagged cargo to the vessel and opening bags into the holds. If there are any signs of firm caking before loading, we would advise caution; in the recent past we have encountered fertiliser cargoes that were still caked after being loaded from a warehouse onto a truck, followed by a sling and onto a pontoon over the hatch. The caked fertiliser was rendered free-flowing by passing it through a mesh over the hold but re-caked into firm lumps at disport, resulting in a large claim.
Therefore, we would advise if any firm caked1 lumps of cargo are observed at any point during the loading process, a protest is made and the master considers clausing the bills of lading accordingly. Often, lumps or caking seen at outturn are attributed to a suspected observable condition during loading, and if the master has not complained or claused documents, then the vessel may well be criticised. Therefore, we would always recommend the duty officers take photos of the cargo during normal routine inspection – this is highly valuable evidence when dealing with such complaints.
Caking might later be blamed on rain during loading, so it is crucial that accurate logs are kept stating whether there were any periods of rain during the loading, and if the hatches were closed in a timely fashion. Ensuring that hatch covers are watertight and that hatch cover surveys are up to date prior to loading can also assist in defending claims.
In the event of wetting during loading, the wetted areas of cargo must be removed, as much as possible. However, as the water will be spread quickly and be absorbed by the surrounding fertiliser, it will be difficult to remove all of the wetted fertiliser.
Ammonium Nitrate
Ammonium nitrate is a hazardous compound that has the potential to explode upon contact with ignition sources. This explosion risk is more likely to occur when ammonium nitrate is contaminated by organic material such as fuel oil, and/or externally heated, for example by hot work or buried cargo lamps. Some of the biggest cargo-related disasters have been caused by ammonium nitrate, for example in Texas City, Tianjin, and Beirut.
There are four entries in the IMSBC Code for ammonium nitrate and ammonium nitrate-based fertilisers (see box for the IMSBC Code entries). There are numerous other entries of ammonium nitrate cargoes within the IMDG Code; for simplicity, we have used the IMSBC Code entries. The criteria stated in the IMSBC Code for determining which entry a cargo belongs to depends on:
- Percentage ammonium nitrate
- Percentage total combustible organic material calculated as carbon
- The chemical nature of the components besides the ammonium nitrate
- The outcome of UN standard tests such as the self-sustaining decomposition trough test
1 we class firm caking as a lump that does not become free-flowing after being dropped from a height of 1 m

Self-Sustaining Decomposition (SSD)
The decomposition of ammonium nitrate-based fertilisers can be highly exothermic (heat-producing) and violent. Toxic fumes and nitrous oxides (NOx) are produced during the decomposition of ammonium nitrate.
The decomposition of ammonium nitrate is referred to as self-sustaining as whilst initially, a considerable amount of energy is required to initiate the reaction, once the decomposition begins, the energy released (as heat) is enough to support further decomposition, i.e. the reaction is self-sustaining.
As the decomposition reaction doesn’t involve external reagents, such as oxygen, the decomposition cannot be controlled by using inert gases, or restricting oxygen. Instead, cooling the cargo is necessary to stop further decomposition. SSD can be controlled by quenching the reaction with water, whilst opening the hatch covers prevents over-pressurisation. The IMSBC Code states in the event of a fire, copious amounts of water should be used, and the heat source should be isolated.
Decomposing ammonium nitrate in one hold can act as a heat source for adjacent holds, therefore care needs to be taken to prevent SSD from spreading to these. The IMSBC Code states the hatch covers of adjacent holds should be opened to allow for maximum ventilation and dividing bulkheads should be cooled.
The decomposition of ammonium nitrate is complex but thought to initially involve an unfavourable, energy absorbing proton (H+) transfer, which is why heat is required to begin the decomposition. Without a sufficient heat source, there would not be enough energy in the system to begin SSD. Temperatures of around 160-170 °C are thought to be necessary before decomposition could initiate. Example ship-related sources of heat found to have caused SSD in past incidents include energised lights in cargo hold and thermal oil heating pipes.
The propensity of ammonium nitrate-based fertilisers to undergo SSD can also be influenced by the presence of trace levels of transition metals, chlorides or contamination by chemicals or fuel oil.
The IMSBC Code relies on the trough test (UN Manual of Tests Methods Part III, Section 38.2.4) to determine whether nitrates containing fertiliser is capable of undergoing SSD. In this test, the trough (Figure X) is filled with fertiliser and decomposition is initiated at one end of the trough. Around 20 minutes after the removal of the initial heating source, the amount propagation of decomposition is measured. If the decomposition has continued after the removal of the source, then the fertiliser is considered capable of showing SSD behaviour. One major limitation of this test is that in bulk, fertilisers are excellent insulators. Therefore, in the case exposure to external heat sources, such as a light in the hold, the heat is unable to dissipate, causing the temperature of the fertiliser surrounding the heat source to rise. Over an extended period of exposure to the heat source, the temperatures could feasibly reach the point where the fertiliser begins to undergo decomposition.
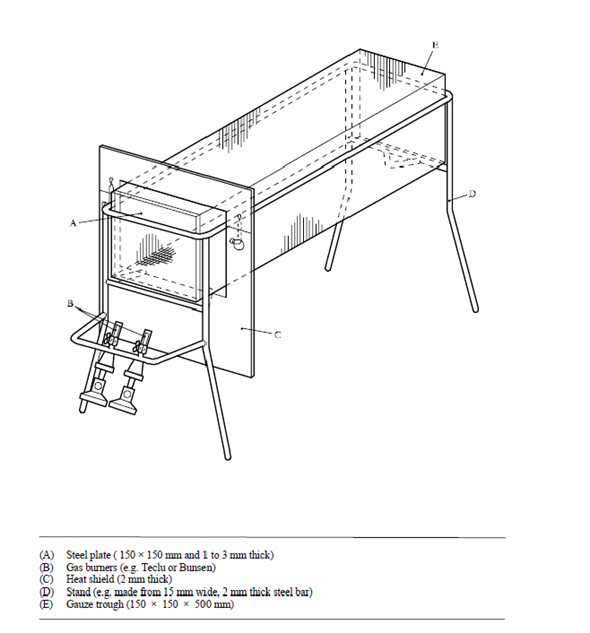
Taken from Manual of Test Methods Part III
Overview : Fertiliser Cargo
A wide range of fertilisers are shipped globally, whilst not all of them have specific hazards associated with them, care must be taken when handling fertilisers Group B cargoes such as ammonium nitrate and potassium nitrate. Using the relevant Code and appropriate cargo declaration is key for eventless transportation by sea. Whereas other fertilisers may not have special hazards related to them, they can nonetheless be involved in incidents such as caking, water damage and contamination.
This article was written by Brookes Bell.
Brookes Bell is a leading multi-disciplinary technical and scientific consultancy serving the marine and energy sectors.
Founded in Liverpool in 1903, Brookes Bell investigates, troubleshoots and advises on a broad range of marine and energy matters and has developed a reputation for being the ‘go-to’ firm for casualty investigation, forensic analysis, technical dispute resolution and expert witness work.
Working across Europe, Asia Pacific and the Americas, Brookes Bell has a global presence allowing the business to provide expert services wherever there is a need.
Alongside the investigative and expert witness services, Brookes Bell operates a state-of-the-art laboratory, The Lab at Brookes Bell, providing the marine, energy, industrial and manufacturing industries with access to world-class forensic analysis and investigative services.
Karwei So
Dr Karwei So is a Managing Scientist at Brookes Bell. She has a DPhil in Materials Science gained at the University of Oxford. Prior to her DPhil, she obtained her Master’s degree in Chemistry at the University of Durham. Karwei is based in Brookes Bell’s Hong Kong office and joined the business in January 2019.
Marcelo Rodrigues
Dr Marcelo Rodrigues is a Managing Scientist at Brookes Bell. He has a BSc degree in Biological Sciences obtained in Brazil, and a PhD in Sciences gained in Spain. He carried out academic research at the postdoctoral level in Austria and the UK. Marcelo is based in Brookes Bell’s Liverpool office and joined the business in May 2018.





